E-Learning
In November 2022, IUHW invited 3 Asian doctors to Japan and gave medical research training over a month. The following movies are excerpts from the training for a self-learning purpose.
Medical Statistics
From a real classroom interactive between lecturer and students, i.e. Asian doctors.
Introduction ~ Statistics in Clinical Research 1 (1:53:00)

Statistics in Clinical Research 2 ~
Data Management in Clinical Research ~ Wrap-Up (slides only) (2:26:00)
Copyright©IUHW, All right reserved
Overview of Clinical Trial
1. Abbreviations in Clinical Trial (14:10)

- Narration Script 1
- Slide Translation (no script)
2. Pharmacokinetics of drugs (08:01)
- Narration Script 2
- Slide Translation (no script)
3. Flow of drug development -Position and role of ARO- (27:35)
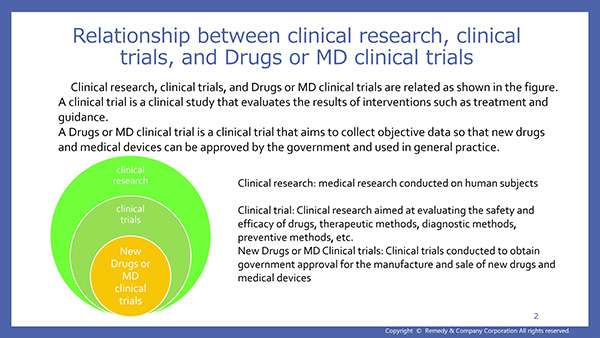
- Narration Script 3
- Slide Translation (no script)
4. Sponsors expect ARO-CRA ideal image (12:49)

- Narration Script 4
- Slide Translation (no script)
5. The relationship between medical institutions and CRA (13:26)
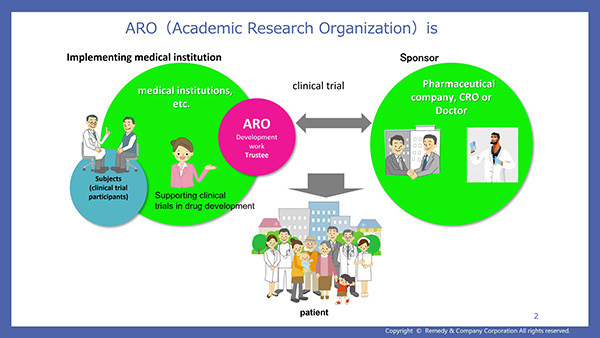
- Narration Script 5
- Slide Translation (no script)
6. Investigational new drug delivery (13:41)
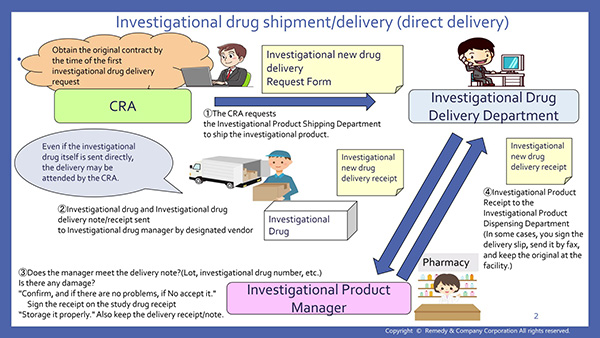
- Narration Script 6
- Slide Translation (no script)
7. 【Procedures during clinical trials 】
Amendments to Clinical Trial Documents (08:25)
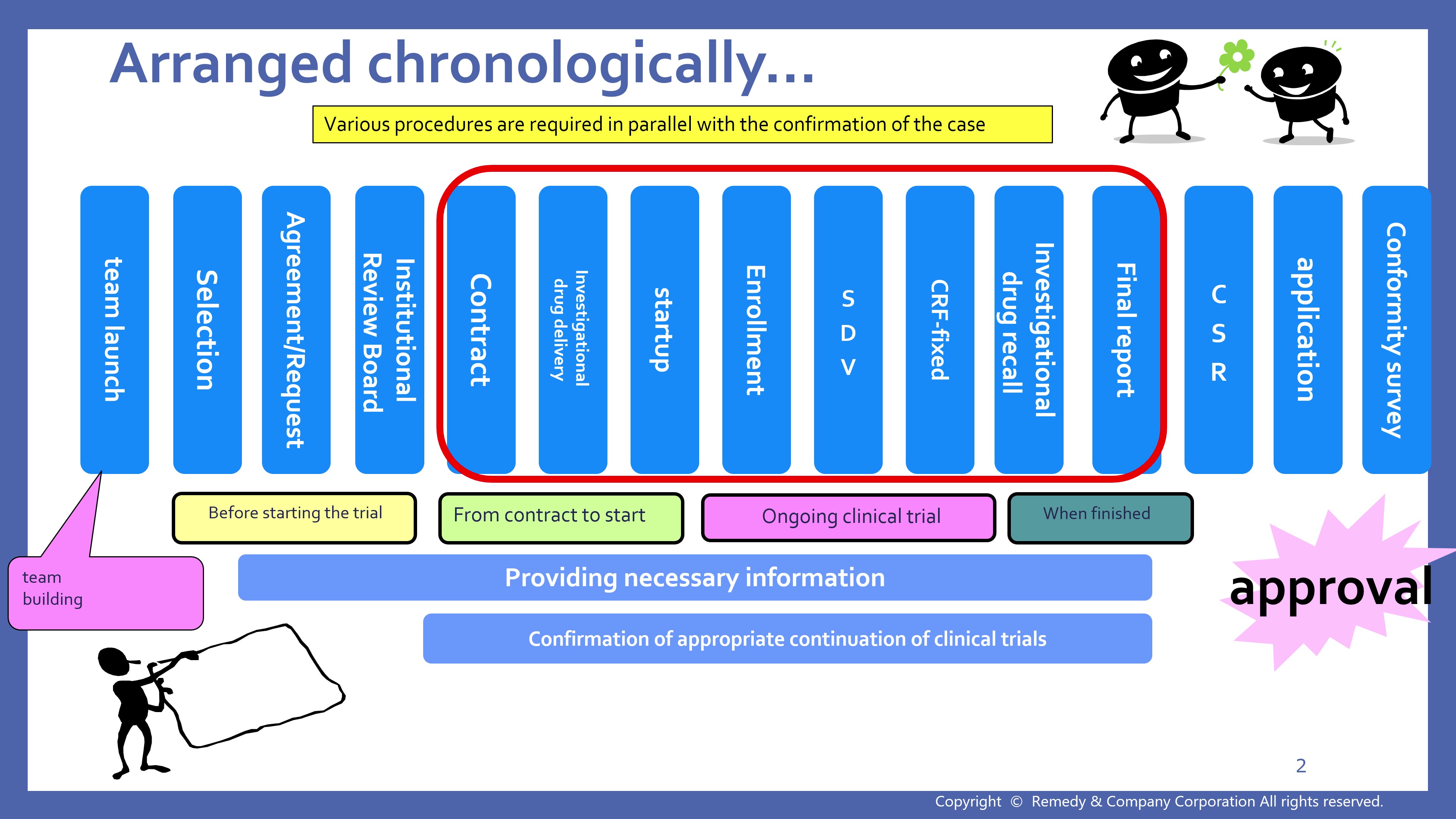
- Narration Script 7
- Slide Translation (no script)
8. Safety Information Monitoring (22:53)

- Narration Script 8
- Slide Translation (no script)
9. Case monitoring guidance (50:11)
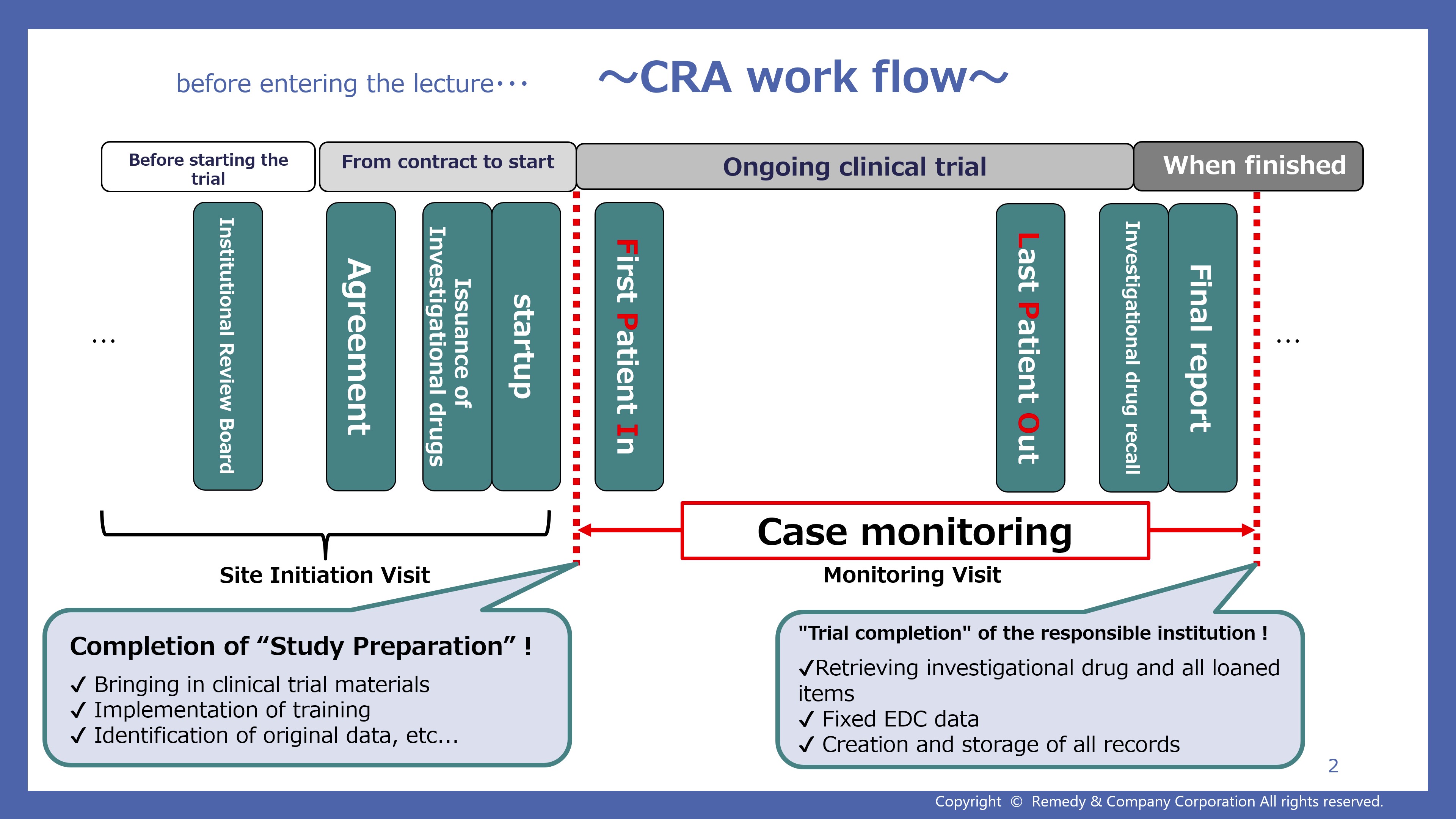
- Narration Script 9
- Slide Translation (no script)
10. Deviation (34:31)

- Narration Script 10
- Slide Translation (no script)

